
INSTI HIV-1/HIV-2 Anitbody Test
The INSTI HIV-1/HIV-2 Antibody Test is a one-time use, rapid, visually read, flow-through immunoassay which detects antibodies to Human Immunodeficiency Virus (HIV) Type 1 and Type 2 using a drop of human fingerstick blood. Other sample types which can be tested are venous whole blood, plasma and serum.

- Test yields results in as little as 60 seconds
- Built-in IgG capture procedural control
- Detects IgM and IgG antibodies
- INSTI works with most testing algorithms
CorDx Malaria Pf/Pv Ag Rapid Test
The Malaria Pf/PV Ag Rapid Test (Cassette) is designed as a simple, rapid, qualitative, and cost-effective method for in vitro testing. This test detects the presence of Pf-HRP-II (histidine-rich protein II) specific to Plasmodium falciparum and pLDH (plasmadicium lactate dehydrogenase) specific to Plasmodium vivax in human whole blood.


Princeton BioMeditech Corporation BioSign Flu A&B
An in vitro rapid qualitative test that detects influenza type A and type B antigens directly from nasal swab, nasopharyngeal swab and nasal aspirate/wash specimens.

- CLIA WAIVED for nasal swab and nasopharyngeal swab specimens
- Rapid diagnosis for early treatment
- Easy-to-read cassette
- Pre-measured extraction reagent capsule for increased accuracy and ease of use
- Detects H1N1 (Swine Flu)

INSTI Hepatits C Antibody Test
The INSTI® HCV Antibody Test is the world’s first one minute test. It is highly accurate, affordable, and easy-to-use. With IgG and IgM procedural control and the ability to test for HCV genotypes 1 – 6, the INSTI HCV Antibody Test is an excellent tool in the fight against Hepatitis C. A hepatitis C blood testing is used to determine if someone has ever been infected with the hepatitis C virus. The INSTI hepatitis C blood tests look for antibodies to the hepatitis C Virus in the blood. They are chemicals that are released into the bloodstream when infected.

- Test yields results in as little as 60 seconds
- Whole Blood/Serum/Plasma
- Sensitivity: 100% - Specificity: 99.7%
- Detects IgM and IgG Antibodies
- INSTI works with most testing algorithms
Princeton BioMeditech Corporation Status Strep A Flip
Rapid Detection of Group A Streptococcal Antigen Directly From Throat Swab Specimens

- External positive and negative controls included
- Single use reagent capsules
- Positive results in 5 min
- Sensitivity 96%, specificity 99%
- No counting reagent drops – fewer steps for greater accuracy
CorDx Influenza A/B + COVID-19/RSV Combo Ag Test
The Influenza A/B+COVID-19/RSV Combo Ag Test (Multi-Panel) is an in vitro immunochromatographic assay for the qualitative and differential detection of nucleocapsid protein antigen from influenza A (including the subtype HIN1), influenza B, RSV and SARS-CoV-2 in nasopharyngeal (NP) fluid specimen.

- Rapid diagnosis of influenza A, influenza B, RSV and SARS-CoV-2 infections
- Multi-Panel display
- For international use
Princeton BioMeditech Corporation BioSign H.pylori IgM/IgG/IgA
For the qualitative detection of anti-Helicobacter pylori antibody in human whole blood, serum, or plasma specimens.

- Requires only 25 μl of whole blood or 10 μl serum/plasma
- Single-reagent procedure
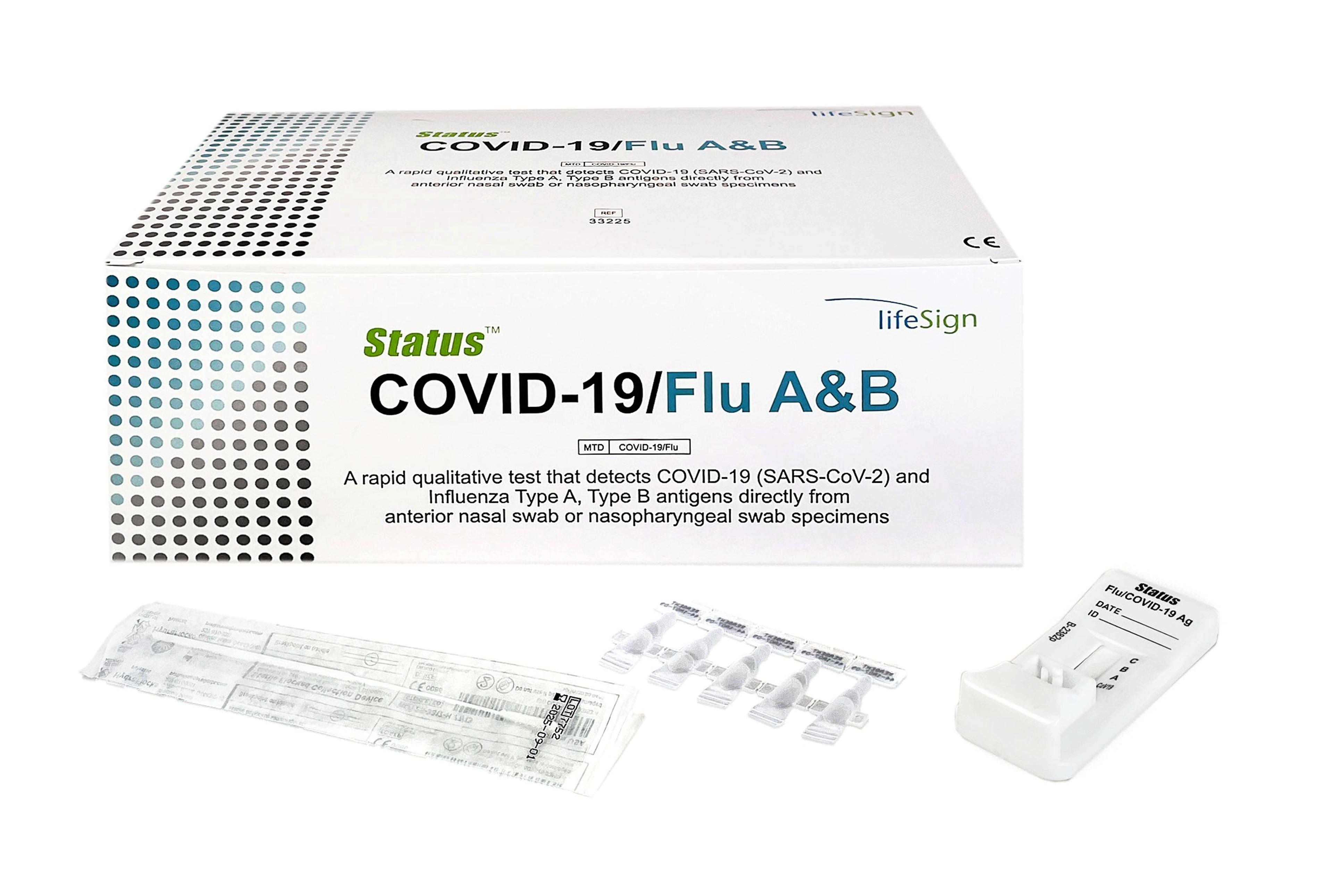
Princeton BioMeditech Corporation Status Covid-19/Flu A&B
A Rapid Immunoassay for the Simultaneous Direct Detection and Differential Diagnosis of SARS-CoV-2, Influenza Type A and Type B Antigen from anterior nasal and nasopharyngeal swab specimens.

- Covid-19: Anterior nasal swab specimen − Sensitivity 93.8 %, Specificity 100%
- Covid-19: Nasopharyngeal − Sensitivity 93.1 %, Specificity 100%
- Flu A: Sensitivity 91.4%, Specificity 95.7%
- Flu B: Sensitivity 87.6%, Specificity 95.9%
- FDA Emergency Use Authorization (EUA)
- Visually read in 15 minutes
- Flocked nasopharyngeal swab for superior specimen collection and patient comfort
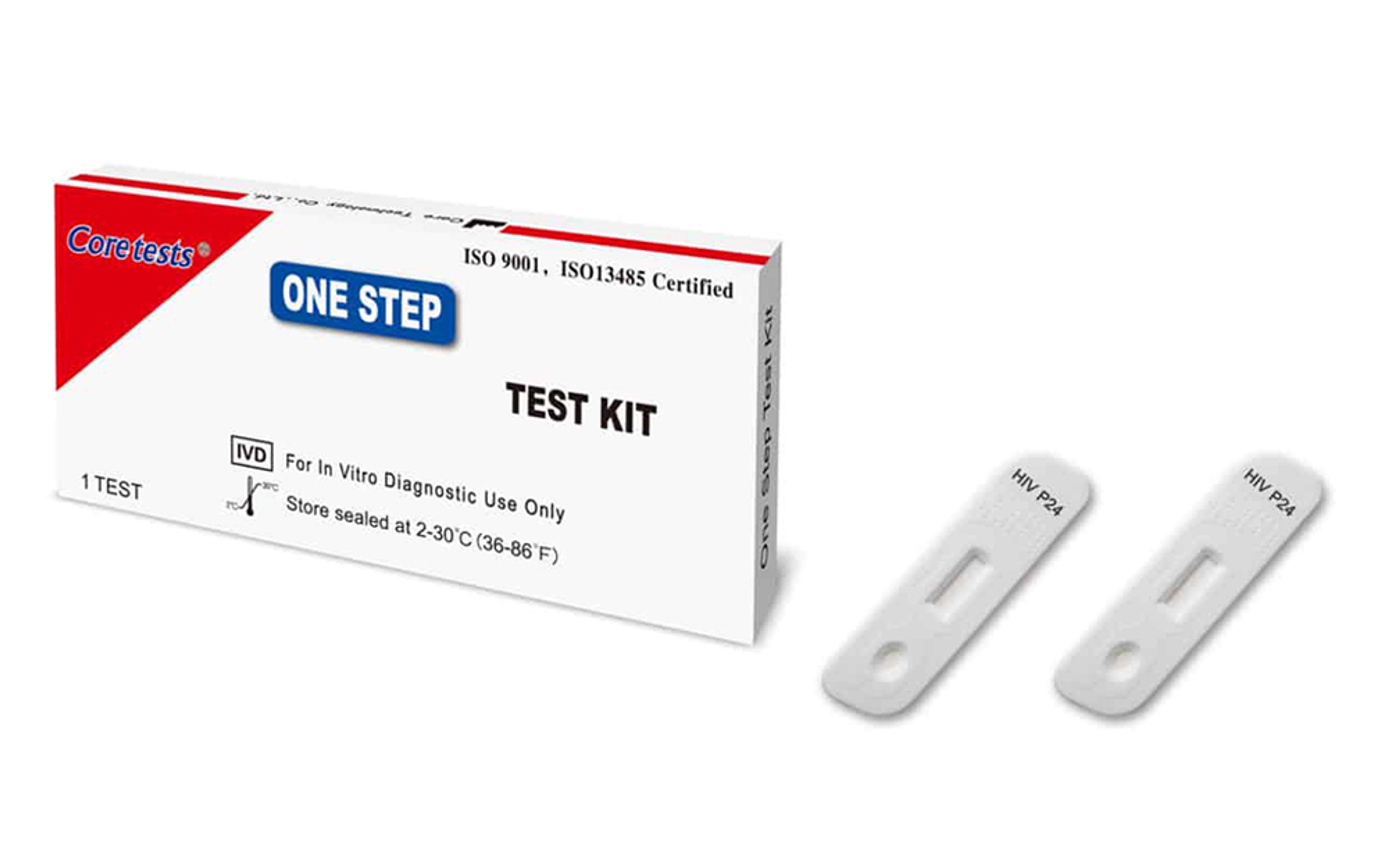
CorDx Whole Blood HIV 1+2 P24 Ag Test
The Whole Blood HIV 1+2 P24 Ag Test (Cassette) is a rapid test for the qualitative detection of Human Immunodeficiency Virus type 1 and type 2 (HIV 1+2) P24 antigens in human whole blood.

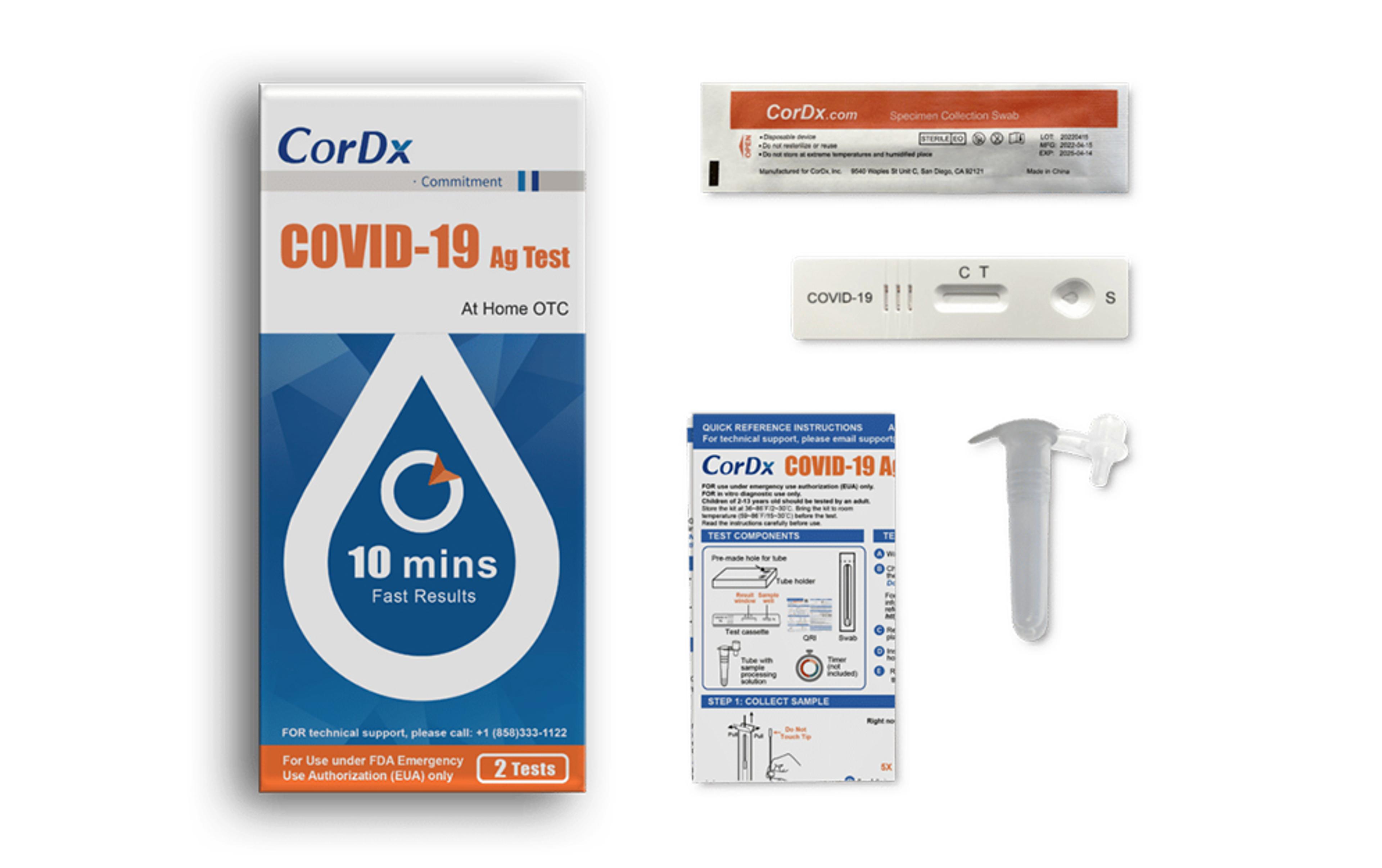
CorDx COVID-19 Ag Test
The CorDx COVID-19 Ag Test is a lateral flow immunoassay device intended for the qualitative detection of nucleocapsid protein antigen from the SARS-CoV-2 virus, delivering results in just 10 minutes.

- Authorized for non-prescription home use with self-collected anterior nasal (nares) swab samples from individuals aged 14 years or older
- Authorized for non-prescription use for adult collected anterior nasal (nares) swab samples from individuals aged two years or older
- This product has not been FDA cleared or approved, but has been authorized by FDA under an EUA
CorDx Hepatitis C Virus (HCV) Test Whole Blood
The HCV Hepatitis C Virus Test Whole Blood (Cassette) is a rapid and convenient immuno-chromatographic assay for the qualitative detection of antibodies to the hepatitis C virus (HCV) in human serum or plasma. It is intended for professional use to aid in the diagnosis of HCV infection. This screening test provides only a preliminary result. All positive results should be confirmed with clinical expertise and professional judgment.

- Sensitivity: 97.9%
- Specificity: 97.9%
CorDx Tuberculosis (TB) Test Whole Blood
The (TB) Tuberculosis Test Whole Blood (Cassette) is a chromatographic immunoassay (CIA) for the direct qualitative detection of tuberculosis antibodies in human whole blood, serum, or plasma.

- Specificity: 94.3%
CorDx Dengue NS1 (DEN) Antigen Test
The Dengue NS1 Ag Test (Cassette) is a qualitative test for detection of the NS1 antigen for the dengue virus inhuman serum, plasma, or whole blood. The test is able to detect the virus on the first day that symptoms are present.

CorDx Group A Streptococcus (GAS) Ag Test
The Group A Streptococcus Ag Test (Cassette) is used for qualitative detection of group A streptococcus antigen from ooropharyngeal or nasopharyngeal swab specimen.


CorDx Monkeypox Virus IgM/IgG Ab Test
CorDx's Monkeypox Virus IgM/IgG Ab Test is a lateral flow chromatographic immunoassay used for the qualitative detection of monkeypox virus IgM and IgG antibodies in human whole blood/serum/plasma samples. The Monkeypox Virus IgM/IgG Ab Test uses colloidal-gold-labeled monkeypox recombinant antigen and mouse IgG, with nitrocellulose membrane fixed with two detection lines and a quality control line.

